


Within the collaborative research project Matrix Evolution scientists from different specialties - from Hannover Medical School (Medizinischen Hochschule Hannover, MHH) and Leibniz University of Hannover (LUH) - are developing complex biomaterials, particularly for regenerative medicine and implant research.
The Lower Saxony Ministry of Science and Culture is funding the research project for 3 years with around 1,5 million euro.
Research in Focus: Biomaterials and Regenerative Medicine
Biomaterials play a key role in regenerative medicine applications. The biophysical and biochemical properties of materials create specific cellular milieus, which have a major influence in cell behaviour and function. As of now, a wide range of natural and synthetic materials are used in the field of regenerative medicine (tissue engineering) and implant research or are being investigated for application. Even though these biomaterials meet important requirements, like biocompatibility, degradability or mechanical stability, they still end up being highly simplified models of the extracellular matrix (ECM). The hierarchical assembly, high compartementalization and dynamic complexity of the real ECM is far from being achieved by the usage of unstructured biomaterials. The in vitro models made from current biomaterials are therefore highly simplified and cannot reproduce the full biological function of tissue. Therefore, there is an urgent need for biomaterials which, like the natural matrix, have a hierarchical structure and complexity and enable the construction of defined architectures from the nano- to the 3D macro-level. The natural ECM is not only highly structured, but also highly dynamic and is remodelled and adapted in physiological processes. For this reason, the Matrix Evolution project also aims to rebuild this dynamic aspect by introducing bioresponsive and temporally switchable elements into the biomaterials. New, bioinspired matrix molecules and materials may lead to an evolution of the matrix in tissue modelling, with significant benefits for all areas of regenerative medicine and clinical research.
MatrixNiche | Prof. Dr. Cornelia Lee-Thedieck
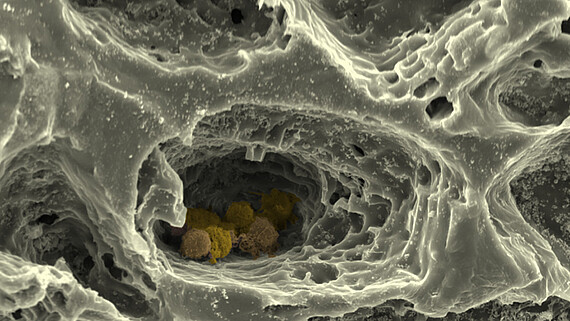
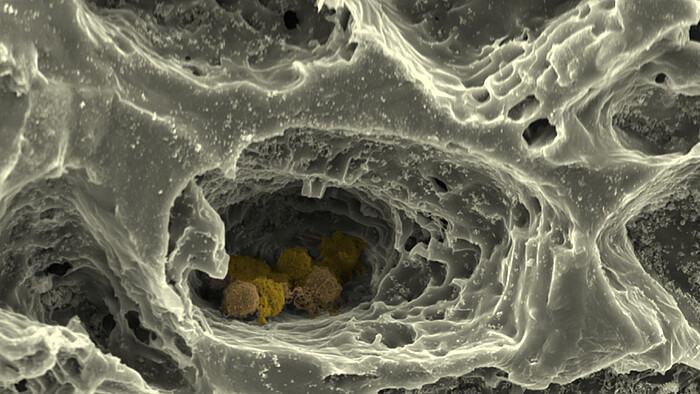
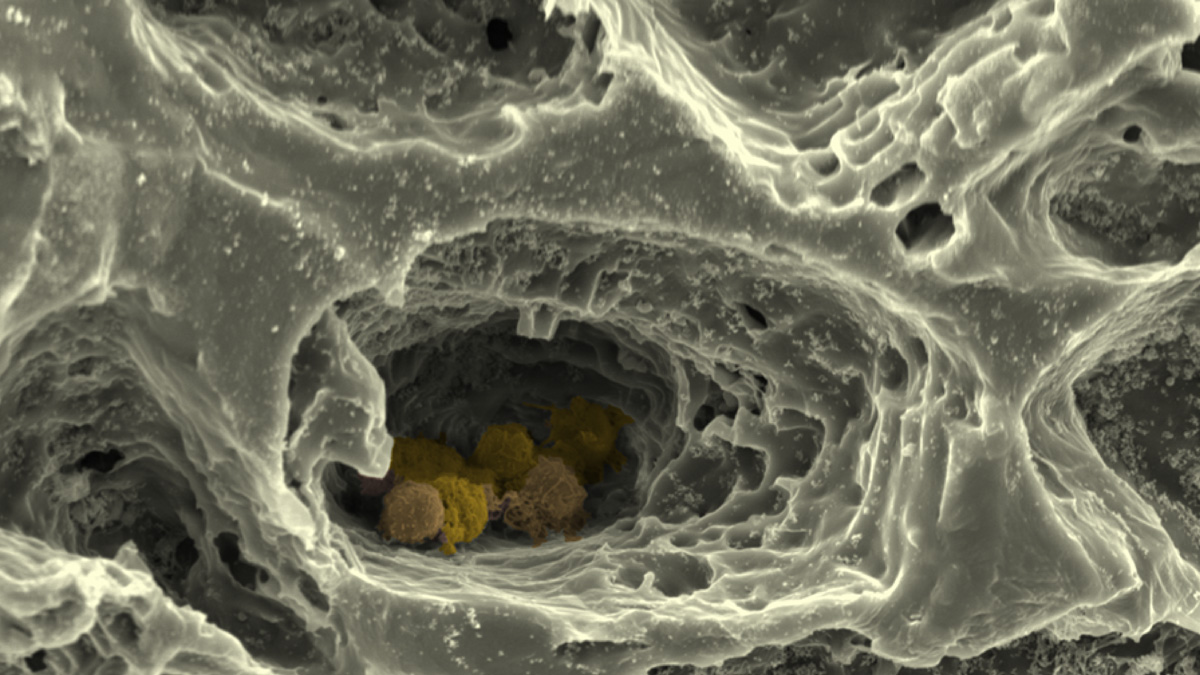
At the Institute of Cell Biology and Biophysics in the Cell Biology section as part of the subproject "MatrixNiche" we mimic the ECM of the bone marrow and the structure of the surrounding bone by combining different matrix molecules in a hierarchically organised, biomimetic biomaterial, which is produced via 3D-printing, electrospinning and crosslinking of polymers to hydrogels. This custom-made matrix allows us to investigate the combined influence of multiple parameters - like nanostructure, macrostructure, stiffness and porosity - on the stem cells of the bone marrow by using super-resolution light- and atomic force microscopy, single cell force spectroscopy alongside state-of-the-art biochemical, cellular- and molecular biological methods. We further use this hierarchically structured matrix in "MatrixNiche" as a scaffold for a multi-cellular, biomimetic in vitro model of the stem cell niche in the bone marrow, which will allow us to analyse the cellular interactions in relation to the matrix properties in the niche.
MatrixBlocks | Prof. Dr. Marie Weinhart



At the Institute of Physical Chemistry and Electrochemistry, Polymers and Biomaterials section, in the scope of the subproject "MatrixBlocks" we are synthesizing cell compatible block copolymers with intrinsic cell adhesive properties. The chemical composition and architecture of the functional polymers are designed in such a way that reversibly linked hydrogels with adjustable mechanical properties can be produced. At the same time, the block copolymer structure enables self-assembly on electrospun network fibres with subsequent covalent bonding to produce cell-adhesive, hierarchically structured synthetic matrices in combination with the reversibly gelling gels for mammalian cells. Using rheological measurements, a quartz crystal microbalance and live-cell microscopy, we are investigating the biodegradability and capacity to model the matrix in the presence and absence of cells in order to optimize it for specific applications in the research network.
MatrixSynBio | Prof. Dr. Selin Kara

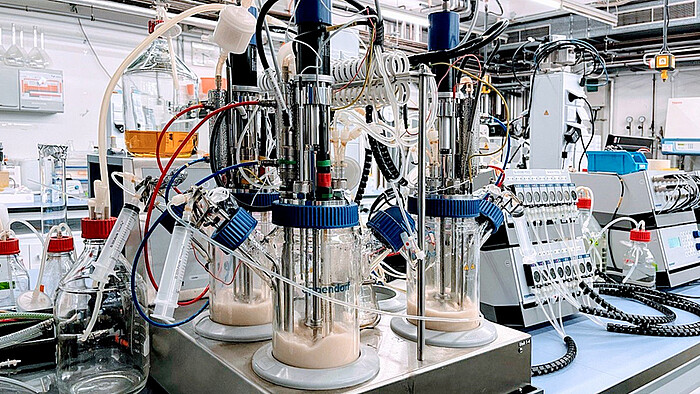
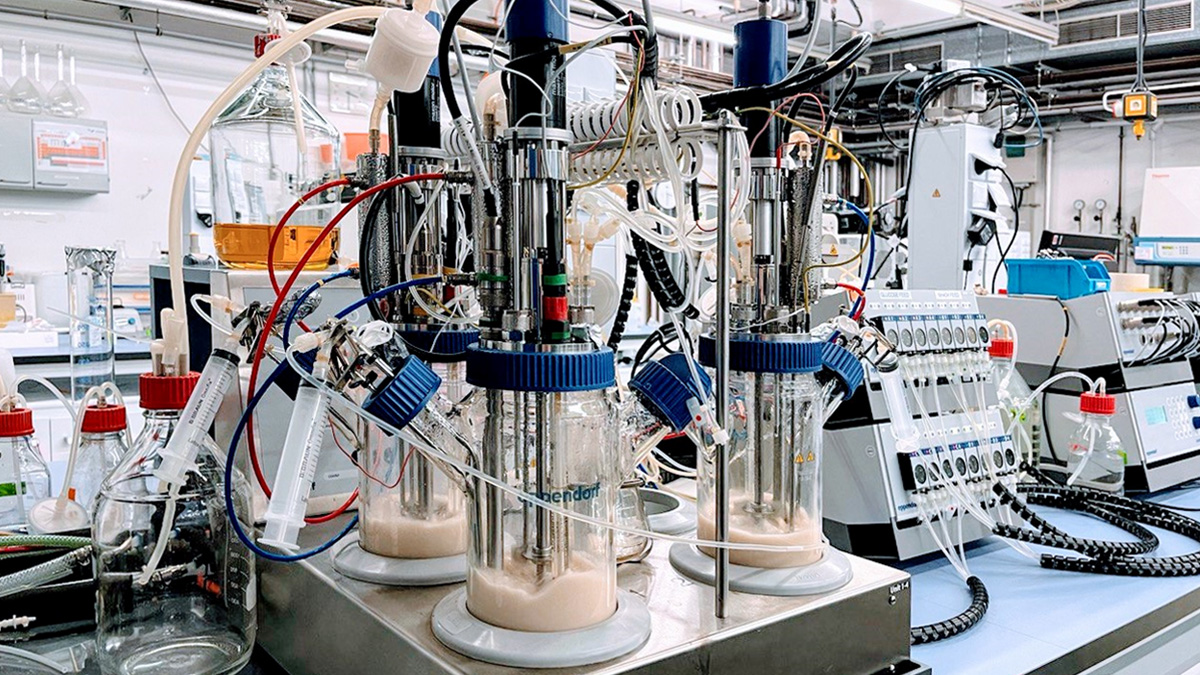
In the working group Design of Biomaterials with Biotechnology, we approach the task of creating a hierarchically structured and dynamic extracellular matrix in MatrixSynBio from the side of protein biomaterials. Our project module is situated at the Institute of Technical Chemistry. Here we use our expertise in bioprocess engineering to produce recombinant segments of the extracellular matrix through microbial fermentation. This enables us to produce biomaterials of consistent quality and reduce the risks of conventional sourcing from animal products. The subsequent generation of hierarchical structures is achieved by chemically modifying the ECM segments, which enables the material to be further processed into desired geometries by electrospinning or bioprinting. Recombinant technology can also be used to reproduce certain aspects of the ECM in the recombinant matrix, such as the ability to bind growth factors and cell adhesion as well as the self-assembly of proteins into higher structures.
MatrixSense | PD Dr. Antonina Lavrentieva

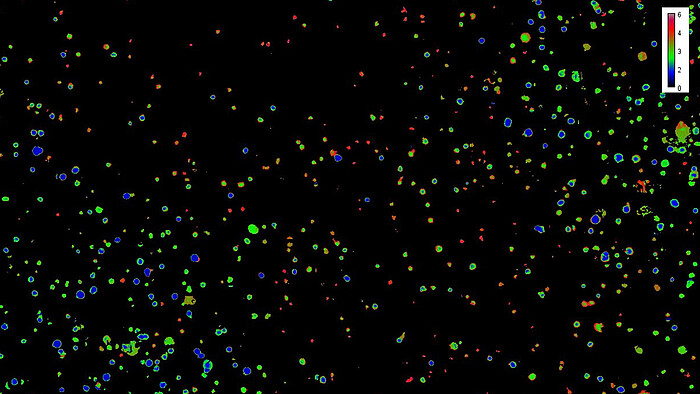
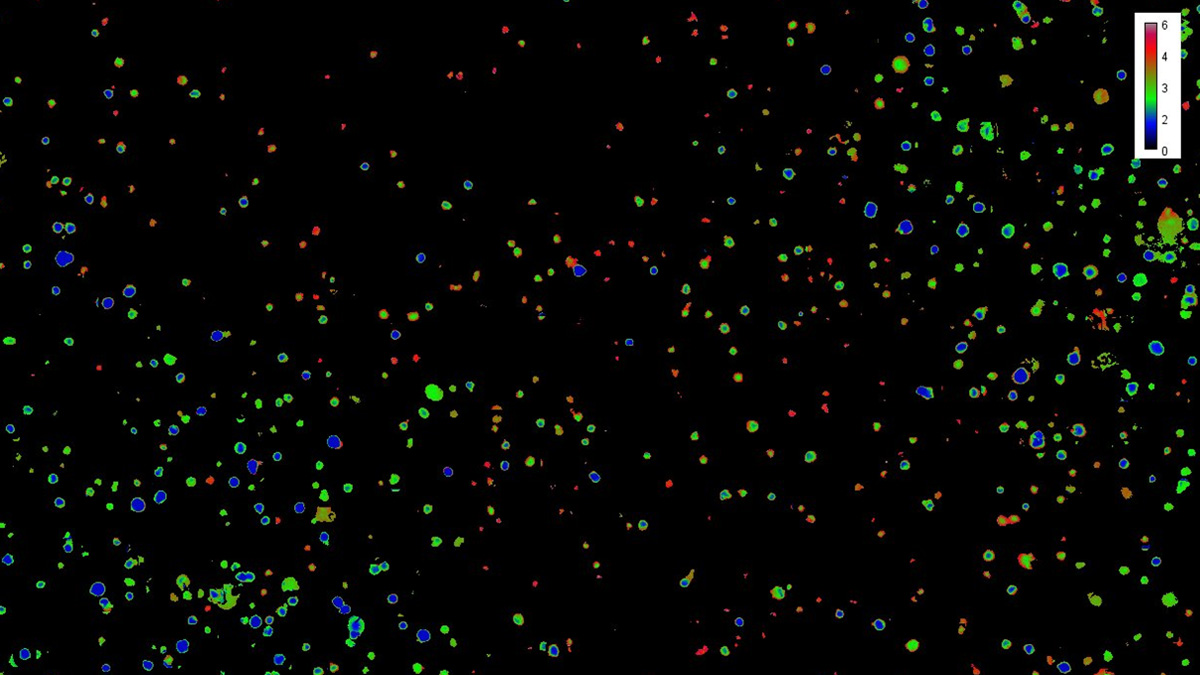
The influence of hierarchical synthetic matrices on cells is usually investigated by endpoint analyses such as cell viability stainings and assays, as well as immunohistochemistry. However, 3D matrix-based cell constructs represent an open dynamic system that is highly sensitive to a variety of factors and stimuli that cells convert into intracellular signals. In the subproject "MatrixSense", which is carried out at the Institute of Technical Chemistry, working group Cell Culture Technology, we have set ourselves these main goals: the integration of genetically encoded biosensors in relevant cell types, characterization of the reporter cells in 2D and 3D cell culture systems and the online evaluation of the spatio-temporal cell response at the single cell level in synthesized matrices. Furthermore, we compare reporter cell reactions with non-invasively measured in situ parameters (e.g. O₂, pH, CO₂). Genetically encoded biosensors based on fluorescent proteins enable real-time monitoring of molecular dynamics in space and time, which is crucial for the evaluation of manufactured biomaterials. We integrate hypoxia-, apoptosis-, Yes-associated protein YAP, actin-, pH- and vinculin-sensors in MSCs and endothelial cells. To monitor cell signalling, we use a novel live-cell analysis system with high-resolution fluorescence imaging, which makes it possible to observe and quantify complex biological changes in real time. Reporter cells are directly applied to the synthesized hierarchical matrices, cell responses are monitored and the correlation between matrix structure and e.g. cytoskeletal structure, hypoxic response, translocation of YAP and expression of vinculin are investigated.
MatrixModel | Prof. Dr. Sophia Rudorf

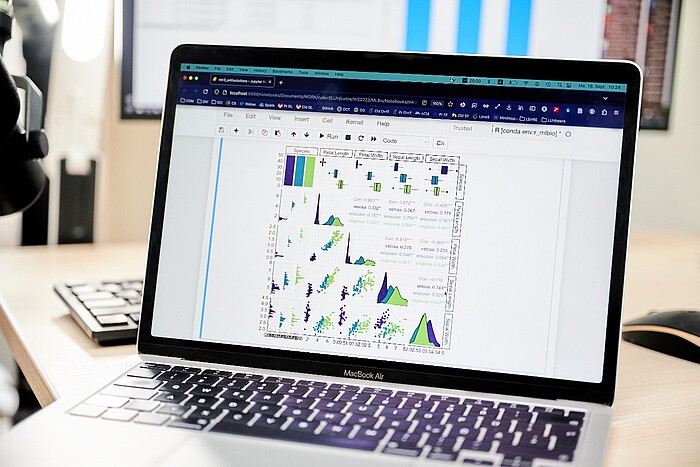
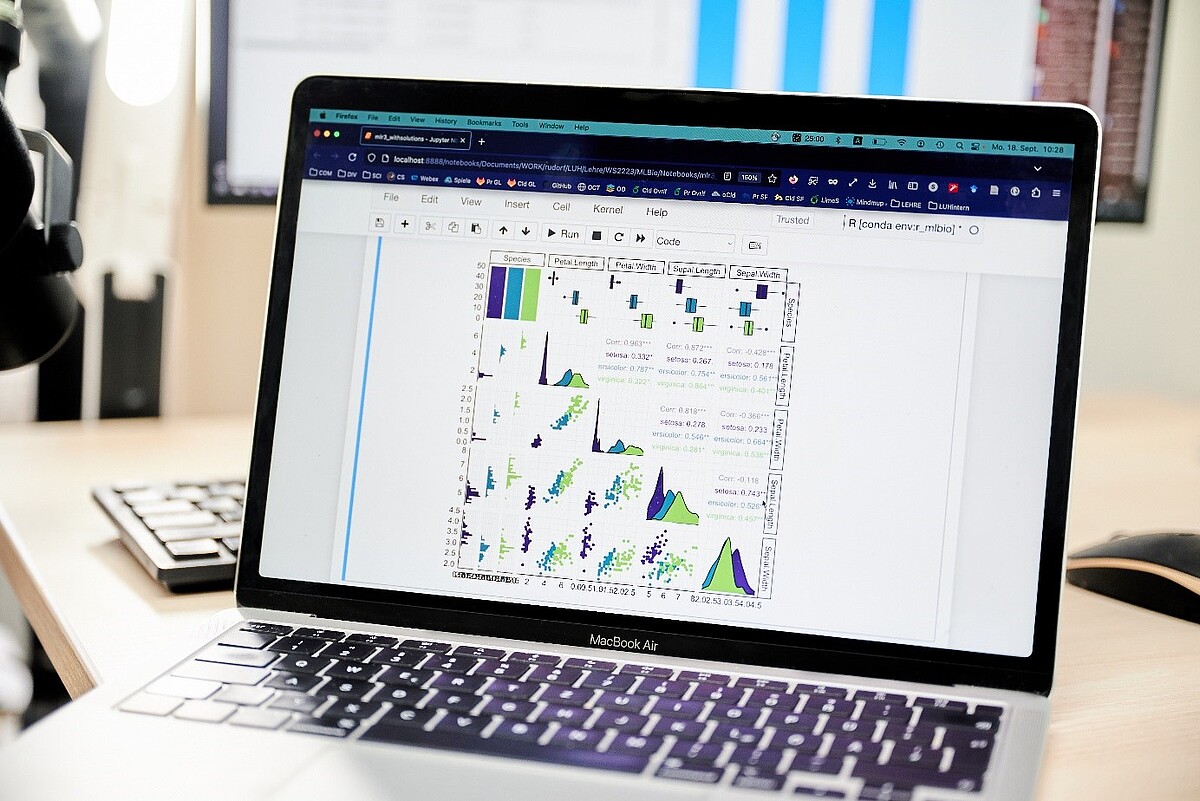
The convergence of experimental findings in quantitative models not only provides a deeper understanding of the process, but can also enable access to information that is inaccessible by experiment. In the Department of Computational Biology of the Institute of Cell Biology and Biophysics, we contribute our experience in the theoretical description and simulation of biomolecular processes within the "MatrixModel" subproject. We use the experimental results from the subprojects MatrixSynBio, MatrixBlock, MatrixSense and MatrixNiche to develop in-silico models of the bioinspired matrices. In particular, we take into account the hierarchical structure of the matrix and the physical-biological interactions between the matrix and different cell types. The models are tested in MatrixModel and the other subprojects as part of prediction-validation cycles and used to optimize the matrices. This enables even more targeted development of the materials.
In addition, we support the synthesis of ECM proteins in microorganisms in the MatrixSynBio subproject with our expertise in the optimization of genetic sequences.
MatrixImplant | Prof. Dr. Meike Stiesch
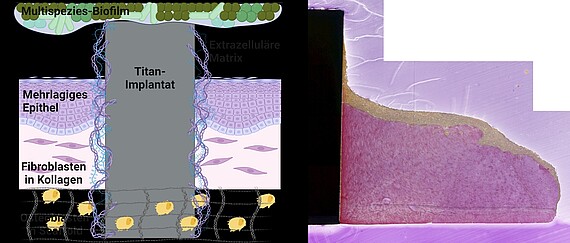
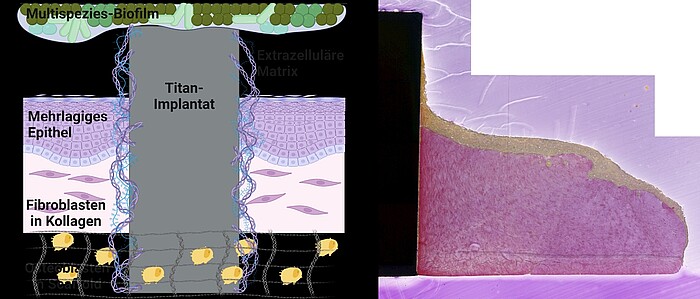
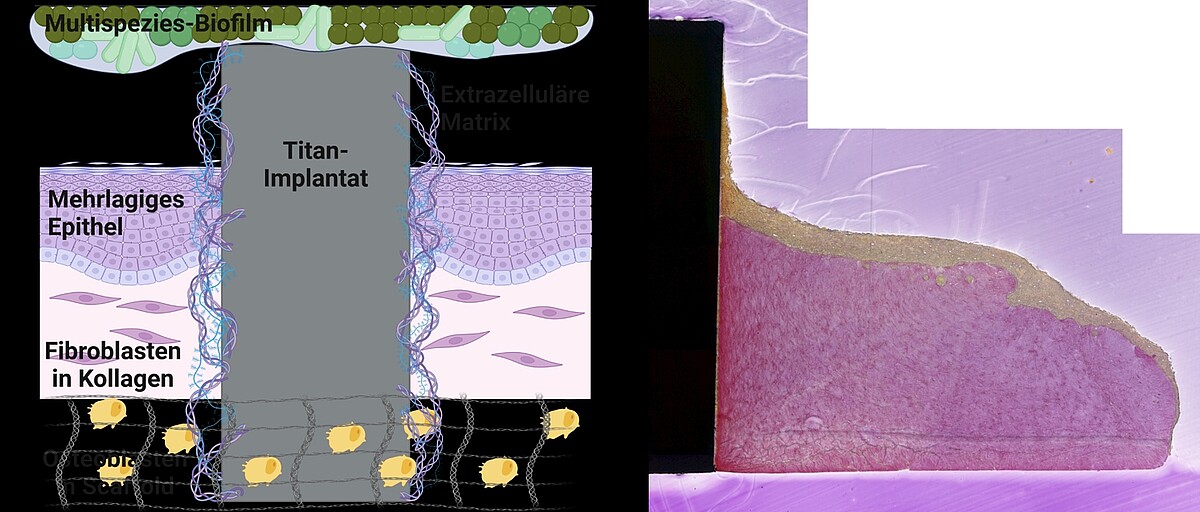
In "MatrixImplant", we are investigating the influence of the extracellular matrix on the development of implant-associated infections that are difficult to treat at the Clinic for Dental Prosthetics and Biomedical Materials Science at Hannover Medical School. To do this, we integrate the structured, recombinant matrix into the complex 3D implant-tissue-oral-bacterial-biofilm (INTERbACT) model. The model consists of an artificial, multi-layered mucosa with an integrated implant in co-culture with an oral multispecies biofilm, and thus reproduces all relevant factors of the peri-implant system. We investigate changes in the implant material caused by the recombinant matrix on a physical and chemical level using atomic force and scanning electron microscopy as well as contact angle measurement. The influence of the matrix on the vitality and integration of peri-implant human cells in the INTERbACT model we analyse by fluorescence microscopy, histology and molecular biology. We are extending the model to include bone tissue in order to investigate the influence of the extracellular matrix on bony healing. We use the results of matrix-material and matrix-cell interactions to analyse the role of the matrix in the infection process and thus develop new approaches for the development of innovative infection-resistant biomaterials.
Funding and Cooperation
Matrix Evolution is funded by zukunft.niedersachsen, a funding programme of the Lower Saxony Ministry of Science and Culture and the Volkswagen Foundation.



Medizinische Hochschule Hannover



Leibniz Universität Hannover



Agenda zukunft.niedersachsen
Speaker Matrix Evolution


30419 Hannover




